FDA Clarifies Purpose of Deficiency Letters Related to Premarket Review of Tobacco Product Applications
Category
Regulatory
U.S. PMTA
SOURCE: FDA Center for Tobacco Products
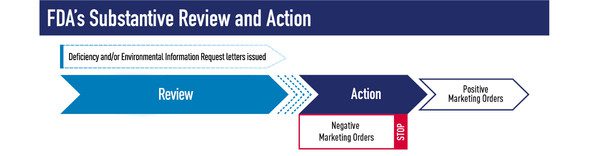
Following the Sept. 9 premarket application deadline for most deemed new tobacco products that are currently on the market, FDA’s job is to process, review, and take action on as many applications as possible before September 9, 2021. As noted in the perspective article FDA issued on Feb. 16, substantive review is the longest and most thorough phase of FDA’s review process because it includes evaluation of the scientific information and data in an application and often includes identification of follow-up questions for the applicant. Before a final decision is made based on substantive review, FDA generally sends a Deficiency letter, which allows one opportunity for applicants to provide additional information that FDA needs to continue its scientific review.
Given that many applicants are experiencing the premarket review process for the first time, and based on FDA’s growing experience with product review and the current review process, FDA recently updated the language in Deficiency letters related to premarket tobacco product applications (PMTAs). The new language clarifies that the intended purpose of such letters is only to communicate information gaps identified during review for which the applicant may wish to provide further information. The language further clarifies that the letter is not intended to convey a list of concerns about the product, and a complete response to the Deficiency letter does not guarantee that the applicant will receive a positive marketing order. Importantly, a final decision regarding marketing of the product(s) will be made at the end of FDA’s scientific review. FDA will base the final decision on the applicable public health standard in the Federal Food, Drug, and Cosmetic Act after reviewing the totality of all the information included in the original submission and amendments.


