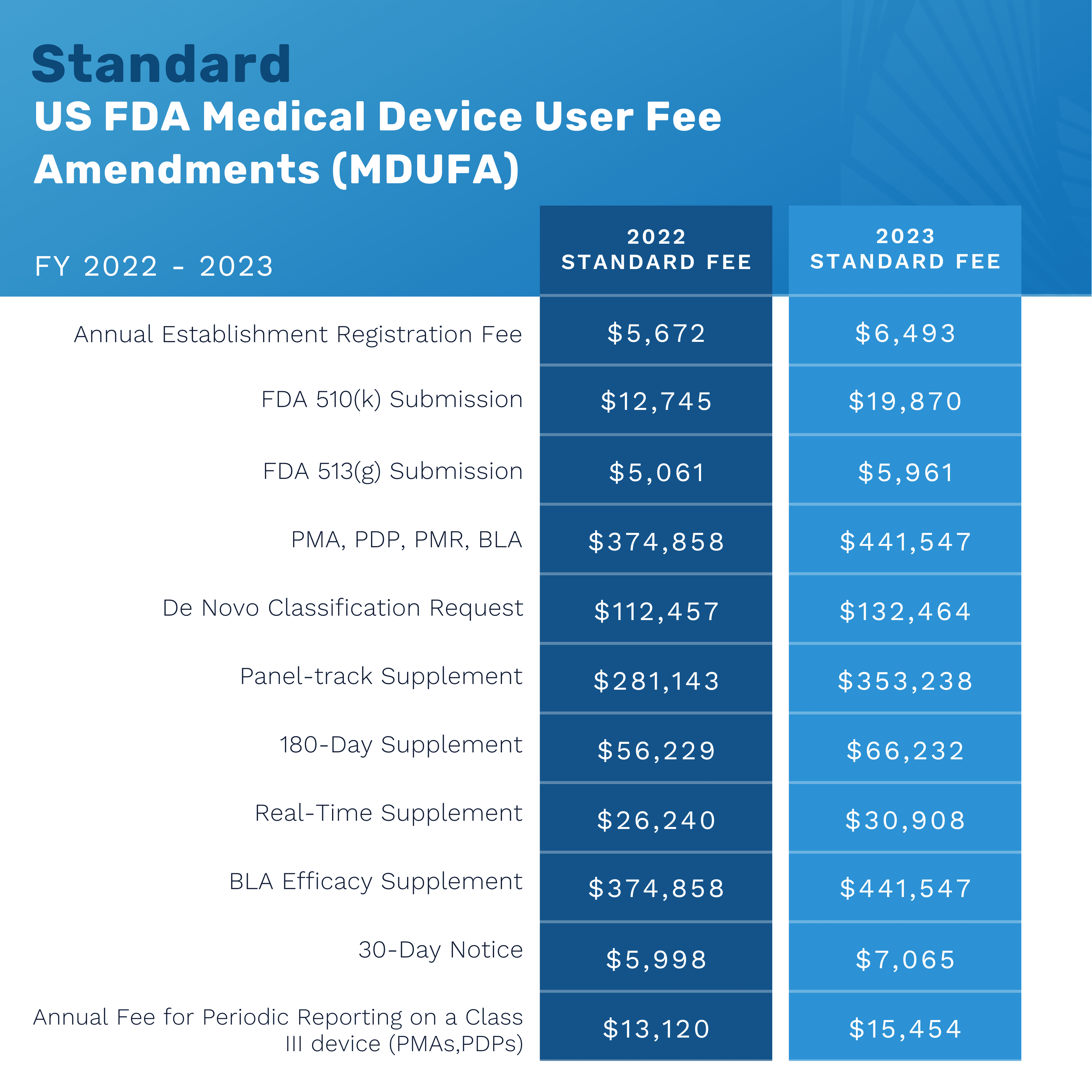
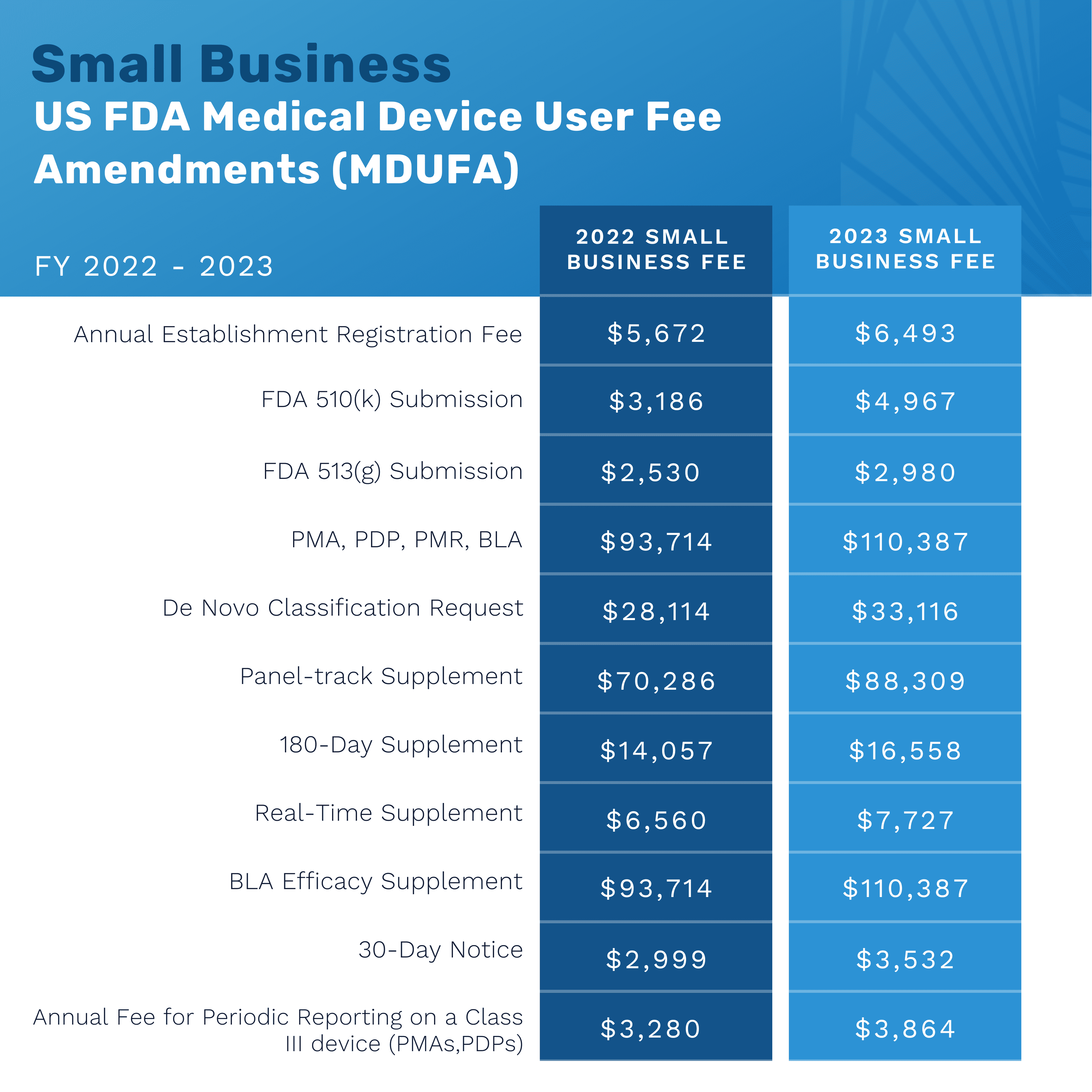
FDA Medical Device User Fees FY 2023
The US Food and Drug Administration (FDA) increased their medical device user application fees for the 2023 fiscal year. Federal law authorizes the FDA to charge a fee for medical device product review and establishment registration. The FY2023 user fees apply to medical device submissions received by the FDA between October 1, 2022, and September 30, 2023. The below graphics show the monetary increase from the 2022-2023 fiscal years for both standard and small business applications.