July 30, 2024 – The following article was developed by Inter Scientific CEO, David Lawson, in collaboration with Accorto’s Cheif Science Officer, Dr. Vince Angelico
Introduction
Those familiar with the Premarket Tobacco Product (PMTA) pathway, the regulatory pathway new tobacco products are required to follow to gain access to the US market, will be equally familiar with the term Appropriate for the Protection of Public Health (APPH). FDA often refers to APPH as the standard for acceptance, yet there is no definition for APPH nor what is expected of it. With the authorization of NJOY Daily Menthol, we have now seen a considerable shift in FDA signaling with respect to APPH and what the agency has set as the standard for vape products.
Background
Section 910 of the Food, Drug and Cosmetics Act requires FDA to make a determination as to the APPH for any tobacco product submitted under the PMTA pathway on the basis of:
a) the increased or decreased likelihood that existing users of tobacco products will stop using such products;
b) the increased or decreased likelihood that those who do not use tobacco products will start using such products
Until recently, it has been up to applicants to guess FDA’s exact requirements for meeting this standard despite FDA issuance Guidance to Industry in March 2023. With FDA now authorizing the first ‘flavored’ ENDS, insights into the review process and the data which tipped FDA’s risk balance now shine a light on the standard that is ‘APPH’.
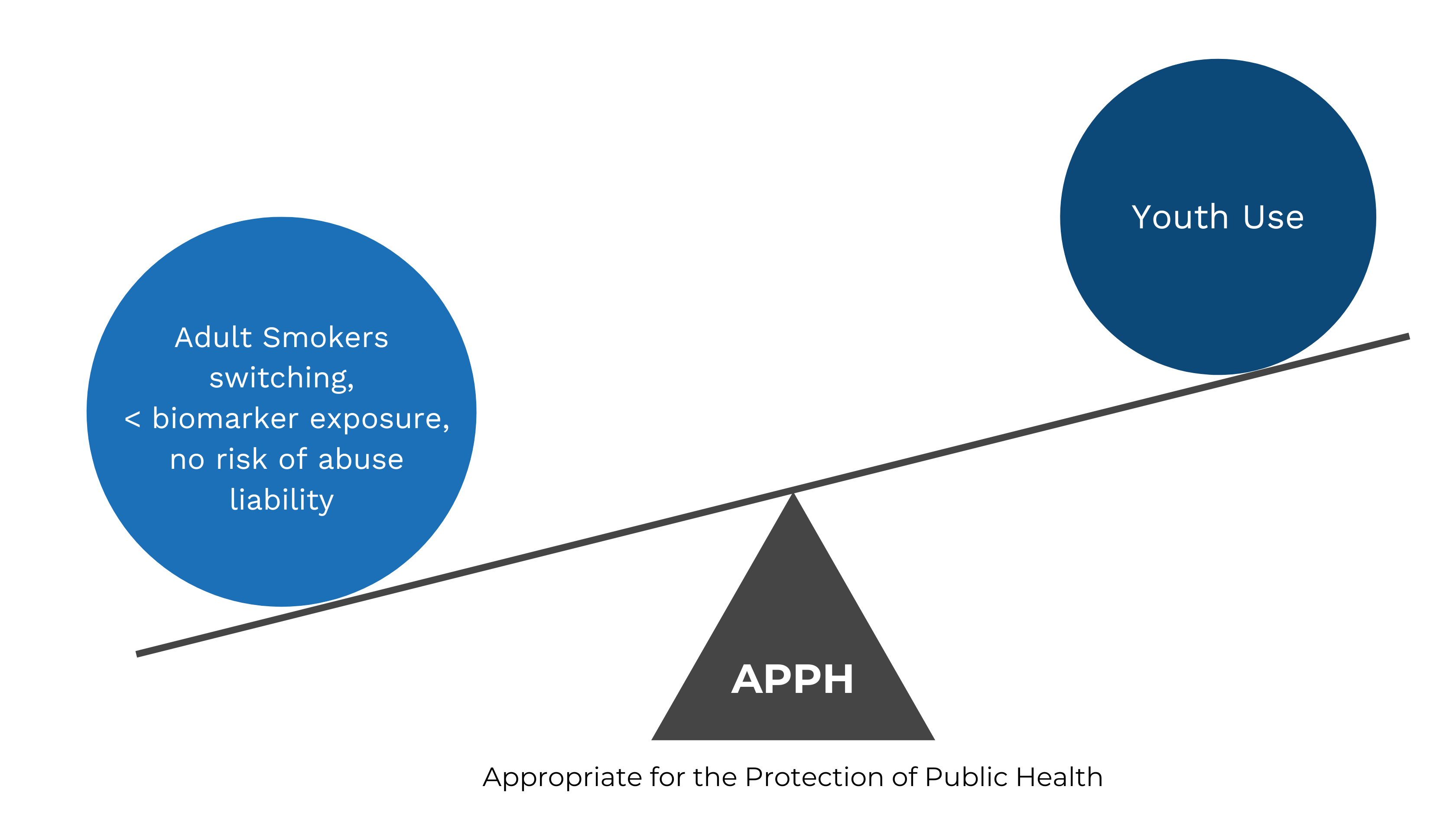
We will now consider how the standard of APPH can be met for ENDS products and how FDA is weighing the risks to non-tobacco users against the benefits of the new tobacco product to adult smokers. This is achieved by balancing the increased risk of non-tobacco users using the new tobacco product against the increased benefit to existing adult smokers from the new tobacco product.
Increased Risk of Non-Tobacco Users Using New Tobacco Products
Despite data highlighting several vulnerable groups who are prone to tobacco use and health inequalities including; young adults, those with lower household income and educational attainment, certain racial or ethnic populations, individuals who identify as LGBTQ+, underserved rural populations, those pregnant or trying to become pregnant, those in the military or veterans, or those with mental health conditions or substance use disorders1,2,3,4,5,6 FDA’s sole concern with respect to ENDS appears to be around the use by minors.
In the case of NJOY Daily Menthol, FDA acknowledges the higher risk of youth use of menthol-flavored ENDS, the risk of youth use of NJOY Daily Menthol is higher than that for Tobacco flavor (20.1% vs 6.4% among high and middle school students) which is based on National Youth Tobacco Survey. The figures for other flavors are substantially higher than that for menthol flavor as cited by FDA.
Further, in its review of the NJOY Daily Menthol PMTA, FDA identified that NJOY is the 10th most-reported brand used in the last 30 days by middle and high school students.
FDA has set a benchmark of tobacco flavor as being the de facto standard for ENDS for which any product seeking authorization must demonstrate substantial benefit over a tobacco flavor product in order to meet APPH.

Table 1. 2023 NTYS Survey data of flavor prevalence amount minors
Increased Benefit to Current Adult Smokers
In order to outweigh the risk of use by minors, FDA requires the applicant to demonstrate a ‘substantial’ benefit to adult smokers. The scale of benefit is thought to be proportionate to the risk posed to minors. For example, based on Table 1 above, in order for a fruit flavor to be authorized by FDA, it would need to be of significantly greater benefit to adult smokers than a menthol flavor at helping the adult smoker reduce or quit combustible tobacco use. In its determination of benefit to adult smokers, FDA considers the following aspects:
- The risk of addiction
- The reduction in smoking combustible cigarettes
- The lifetime cancer risk
The Risk of Addiction
The risk of addiction of a new tobacco product, or abuse liability as it is sometimes called, is a comparison of the new tobacco product’s nicotine delivery (and potentially other compounds) against that of a combustible cigarette. For products delivering too little nicotine, FDA has raised concerns about the potential benefit to adult smokers in helping them transition from combustible cigarettes. Ideally, a new tobacco product will deliver a similar amount of nicotine to that of a combustible cigarette in order for it to effectively reduce or stop adult smokers from smoking.
For new tobacco products that deliver more nicotine, FDA will likely raise concerns about potential addictive properties of the new tobacco product which would not meet an APPH standard.
The Reduction in Smoking Combustible cigarettes
The essential tool in the PMTA application to demonstrate benefit to adult smokers is data supporting the ability of the new tobacco product to either substantially reduce the amount of combustible cigarettes consumed by an adult smoker or the complete switch from combustible tobacco use to ENDS use. Substantial reduction is understood to mean a >50% reduction in cigarette consumption.
Data from two types of studies are typically considered:
- Randomized controlled trials are clinical investigations where subjects are randomly assigned one or more new tobacco products to evaluate the effect of the new tobacco product on their ability to reduce or completely switch
- Longitudinal cohort studies are studies lasting typically >6 weeks where subjects are observed with typically two or more groups e.g. tobacco and non-tobacco-flavored ENDS. These studies may be conducted as self-reported studies via online surveys.
Lifetime Cancer Risk
FDA assesses the potential lifetime cancer risk on an ingredient or compound basis when determining the lifetime risk of the new tobacco product use. FDA has recently published a memo8 on a standardized approach to addressing lifetime cancer risk. In its assessment, FDA considers; ingredients, harmful and potentially harmful constituents, and leachables. It is recognized that ENDS commonly contain genotoxic compounds such as acetaldehyde and formaldehyde and by design contain nicotine which is cytotoxic. FDA is primarily concerned with the lifetime cancer risk of the new tobacco product when compared against other authorized ENDS and combustible cigarettes.
In its authorization of NJOY Daily Menthol, FDA considered the absolute quitting rates of subjects on a longitudinal cohort study which were as follows:
- Tobacco flavor complete switching rate is 21-37%
- Menthol flavor complete switching rates 32-43%
The substantial increase in complete switching for menthol flavor is substantially higher than that of tobacco flavor, greatly benefiting adult smokers. FDA further considers that clinically NJOY Daily delivers a similar amount of nicotine as combustible cigarettes raising no concerns of abuse liability. Finally, FDA highlights that there is a reduced biomarker exposure and HPHC exposure vs cigarettes, which is to be expected for any ENDS.
Determining APPH
Despite highlighting the increased risk of use by minors of non-tobacco flavored ENDS, such as menthol flavor NJOY Daily, FDA must consider the risk to minors vs. the benefits to adult smokers. As there is no increased risk of abuse liability identified and as the biomarker exposure from ENDS is significantly lower than that for combustible cigarettes, the primary determination as to whether NJOY Daily Menthol is APPH, considering that NJOY Daily Tobacco has been authorized, is whether the increased risk to youth use is overweighed by the benefit to adult smokers.
FDA summarizes their view succulently in the TPL for NJOY Daily Menthol as follows:
“Together, the available evidence suggests that although the menthol‐flavored new products pose risks to youth, the potential of the new products for adults who smoke CC to promote cessation and provide significantly lower health risks than CC outweighs that youth risk.”7
As such, in the case of NJOY Daily Menthol, the increased benefit to adult smokers completely switching from combustible cigarettes to NJOY Daily Menthol outweighed the risks of use by minors.
Conclusion
For any application submitted to FDA for a non-tobacco flavor, it is evident that it is key to demonstrate that the new tobacco product has to substantially benefit the adult smoker, over that of the benefits of a tobacco-flavored ENDS. The degree of additional benefit over that of a tobacco-flavored product is unclear and is likely to be proportionate to the increased risk that the group of flavors is seen to have on the risk of use by minors.
References:
1) HHS, “The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General.” Atlanta, GA: HHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Available at: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf
2) HHS, “Tobacco Use Among U.S. Racial/ Ethnic Minority Groups–African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General.” Atlanta, GA: HHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1998. Available at: https://www.cdc.gov/tobacco/data_statistics/sgr/1998/complete_report/pdfs/complete_report.pdf ;
3) NCI, “A Socioecological Approach to Addressing Tobacco-Related Health Disparities,” National Cancer Institute Tobacco Control Monograph 22. NIH Publication No. 17-CA-8035A. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017. Available at: https://cancercontrol.cancer.gov/sites/default/files/2020-08/m22_complete.pdf
4) HHS, “National Stakeholder Strategy for Achieving Health Equity,” April 8, 2011. Available at: https://www.phdmc.org/programdocuments/healthy-lifestyles/dche/64-achieving-health-equity/file
5) ; U.S. National Institute of Drug Abuse. “Tobacco, Nicotine and E-Cigarettes Research Report,” June 9, 2020. Available at: https://www.drugabuse.gov/download/1344/tobacco-nicotine-e-cigarettes-researchreport.pdf?v=4b566e8f4994f24caa650ee93b59ec41
6) ; HHS, “Preventing Tobacco Use Among Youth and Young Adults,” A Report of the Surgeon General, 2012. Available at: https://www.hhs.gov/surgeongeneral/reports-andpublications/tobacco/index.html.
7) FDA, Technical Project Lead (TPL) Review of PMTAs. NJOY LLC ENDS. June 21, 2024
8) FDA, Genotoxicity Hazard Identification and Carcinogenicity Tiering of Constituents in ENDS Premarket Tobacco Product Applications. Not Published. June 3, 2024.


